MCAZ Microbiology Laboratory is responsible for microbiological quality control of medicinal products and allied substances. The laboratory was recently upgraded to a state-of-the-art laboratory to meet WHO Prequalification (PQ) requirements for Pharmaceutical Microbiology Laboratories.
A. Pharmaceutical Microbiology Testing
The laboratory carries out tests in accordance with International Standards and Phamacopoiea such as BP, USP, European Pharmacopoeia and WHO guidelines.
The following tests are performed in the Microbiology Laboratory:
- Sterility
The laboratory has a state-of-the-art, purpose-built Cleanroom incorporating a Grade A environment (Class II Biological Safety Cabinet) with a Grade B background (Sterility Room). Sterility testing of samples is carried out in the Biological Safety Cabinet thereby minimizing the potential of false positive results.
Two methods are used to perform the Sterility test:
(i) Membrane Filtration
(ii) Direct Inoculation.
- Microbiological Examination of Non-Sterile Products (MENSP)
Microbial contamination testing of non-sterile products is performed using pharmacopeial or client-supplied methods to determine the bioburden within the sample. Microbial enumeration – Total Aerobic Microbial Count (TAMC) and Total Yeast and Mould Count (TYMC) analysis and Tests for Specified Microorganisms are employed for this test.
- Bioburden Determination
Bioburden testing is performed to ensure that products comply with the specified standards.
Bioburden testing is used in a number of situations such as:
(i) validation and revalidation of sterilization processes
(ii) routine monitoring for control of manufacturing processes
(iii) assessment of the efficiency of cleaning processes.
- Antimicrobial Efficacy Testing (AET)
The laboratory performs Antimicrobial Effectiveness Testing (AET) or Preservative Effectiveness Test (PET) on an array of products. The product is inoculated with a specified number of challenge microorganisms and examined over a period of 28 days, to determine the number of viable microorganisms that survive.
- Bacterial Endotoxins Test (LAL method)
The laboratory can perform analysis of endotoxins using the Gel Clot method. Limulus Amoebocyte Lysate (LAL) is used to detect bacterial endotoxins extracted from the products by forming a gel clot.
- Microbial Identification by Vitek 2 XL Microbial Identification System
The laboratory can identify bacterial and fungal isolates from various processes using a state-of-the-art Vitek 2 XL Microbial Identification System which complements the traditional Gram stain and microscopy techniques. The laboratory is able to identify a vast library of environmental and clinical microorganisms to aid in background environmental analysis, failure investigations and contamination issues.
- Microbiological Assay of Antibiotics
The microbiological assay of an antibiotic is based on comparison of the inhibition of growth of sensitive micro-organisms by measured concentrations of the antibiotics under examination with that produced by known concentrations of a standard preparation of the antibiotic having a known activity.
Microbiology Laboratory estimates the activity of antibiotics by Agar Diffusion method in wells.
B. Training on Pharmaceutical Microbiology Testing
The laboratory offers training on pharmaceutical microbiology testing to laboratory scientists. The scientists are trained on the above-mentioned tests and microbiological basic techniques such as serial dilutions, plate pouring, streaking, colony counting, Gram staining, microscopy, aseptic techniques etc.
C. Service Delivery Time
The laboratory commits to a Turnaround Time of 30 working days for sample received for analysis. However, the laboratory makes efforts to ensure that results and Certificates of Analysis are produced in the shortest possible time.
D. Sample Submission Document
Guidance on Sample Size Requirements for Microbiology Laboratory Analysis
Guidance on Sample Size Requirements
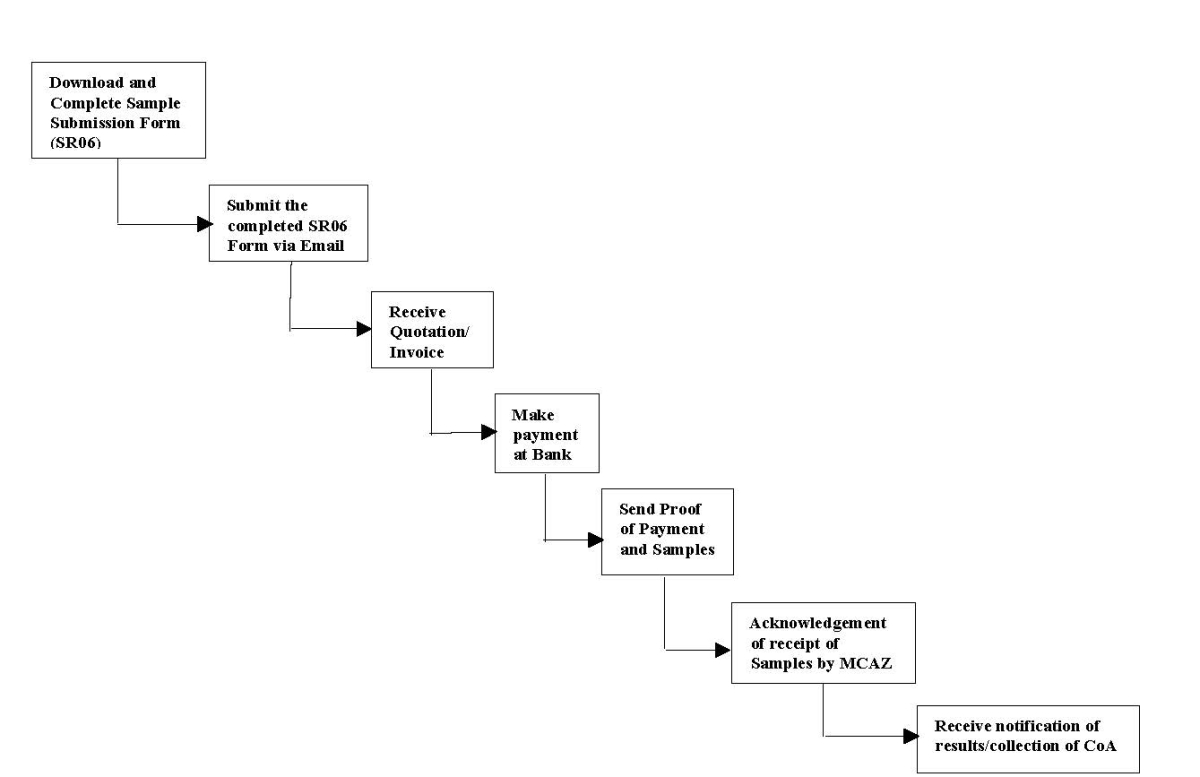
E. Sample Submission Process Flow
*30 working days service delivery (TAT) from date of submission of samples
F. QA activities
The laboratory regularly participates in Proficiency Testing (PT) schemes. These PT schemes are used for benchmarking laboratories performance with regional and international trends in Microbiological Quality Control testing of medicines.