The MCAZ Chemistry laboratory is responsible for the chemical analysis of medicinal products and allied substances. It is an ISO/IEC 17025 Accredited and WHO Prequalified Laboratory which has also been designated as a Regional Centre of Regulatory Excellence (RCoRE) by the New Partnership for Africa’s Development (NEPAD).
WHO Prequalification combined with ISO 17025 accreditation places the Chemistry Laboratory in a strategic position to contribute immensely Locally and globally through the provision of excellent medicine quality control services.
Scope
The laboratory performs comprehensive analytical work on oral dosage forms, oral liquids, sterile injectables, Active Pharmaceutical Ingredients (APIs) and other investigational samples using verified analytical methods to ensure compliance with global quality assurance benchmarks.
Scope of accreditation and pre-qualification includes:
-
-
-
- Identification by FTIR, TLC, UV, and HPLC
- Assay by liquid chromatography, gas chromatography, spectrophotometry, Titration
- Dissolution
- Physical tests pH /friability /Disintegration / Hardness/Thickness/ Diameter
- Loss On Drying
- Moisture determination by Karl Fisher
- Uniformity of Dosage
- Impurities and degradation products /Related substances using HPLC / UV-Vis
-
-
Service Delivery Time.
The laboratory commits to a Turnaround time of 20 working days for sample received for analysis, however the laboratory makes efforts to ensure that results and Certificates of analysis are produced in the shortest time possible.
Sample submission guidelines
-
-
-
- Handling of test items
- Guidance of sample size requirements for laboratory analysis
- Sample submission Flow
-
-
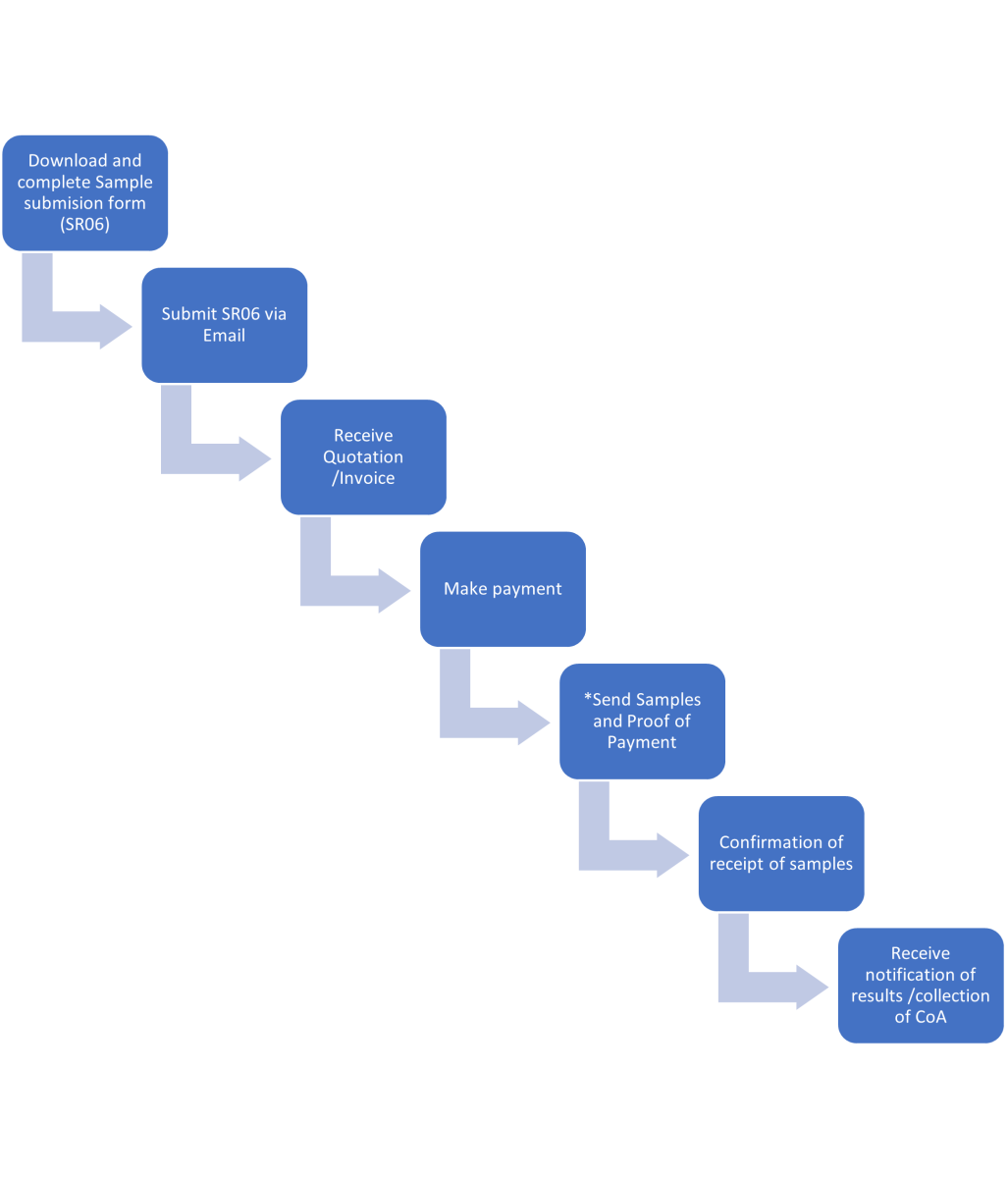
*20 working days service delivery (TAT) from date of submission of samples
Training
The laboratory also offers trainings on laboratory operations and systems to interested customers. Training is conducted at a fee and could be in the form of attachments, short courses, and exchange programs. As part of its RCoRE obligations the Laboratory also carries out Capacity building trainings and mentor-ship for other National Regulatory Agencies (NRAs).
Trainings covers but not limited to the following areas:
-
-
-
-
- Laboratory design and management
- Samples analysis
- Laboratory equipment management
- Inhouse hands-on training
-
-
-
These trainings can be tailor-made to suit the client’s requirements and are available in-person and virtually.
QA activities
The laboratory regularly participates in inter-Lab Proficiency testing (PT) schemes and collaborative testing activities. These PT schemes are used for benchmarking Laboratories performance with regional and international trends in Quality Control testing of medicines.
-
-
-
- Latest PT participation
-
-
